Calcium
Description
This section is from the book "Research In Physiopathology As Basis Of Guided Chemotherapy With Special Application To Cancer", by Emanuel Revici. Also available from amazon: Research In Physiopathology
Calcium
The biological activity of calcium, another member of the II A series and a D inducing element belonging to the cellular level, also is of interest.
In its absorption by grass from the soil, Ca parallels Mg, another D inducing element, but opposes K, an A inducing element. Grass tetany is thus induced by high K and low Ca and Mg values. Ca, which is another D inducing element like copper is antagonistic to zinc, an A inducing element. It has been observed that cancer is less frequent in the so called calcerous clay regions where the soil is formed by limestone. (139) Together with other minerals, an optimum of calcium in soil may help to prevent cancer. While SiO2 favors cancer, Ca appears to prevent it. Calcium also is an antagonist to zinc which, in high doses, seems to favor the development of cancer. (140) The relationship between Ca and K has permitted us to be more precise about the role of Ca in cancer pathogenesis. As opposed to K, which increases by as much as 60% in tumors, the content of calcium decreases by 44%. (141-147)
Confronted with K and Ca changes, it appeared interesting to see to which element we could directly attribute the increase in malignancy. In the regeneration of liver cells, where rapid growth without malignancy takes place, potassium is increased while the amount of calcium is unaltered. Similarly in other rapidly growing but normal cells, calcium is not diminished while K is increased. Potassium thus appears to be related to the process of cellular growth and multiplication which represents also an added factor in transforming noninvasive into invasive cancer. However, potassium is not directly related to the cancerous character of the cells. On the other hand, reduced amounts of calcium appear to be peculiar to the cancerous process. (147) The reduced calcium in cancer is not due to a lack of the element in the organism since calcium is not only available but even apparently present in excess at the systemic level. As we have shown, a high urinary calcium index, indicating exaggerated excretion, is present in the type A offbalance. The anomaly resides in the low capacity of the cancerous cells to fix and properly utilize calcium. As calcium acts at the surface of the cell and its deficiency reduces cellular adhesiveness, (148, 149) lack of cellular calcium can be seen to increase the invasiveness of cancer cells and the tendency to metastases. Deficiency of calcium in cells appears related to the character of youth while excess seems to result in rapid aging.
The anomaly induced by the qualitative deficiency in calcium thus appears to be at the cellular surface. Related to it also are manifestations at the tissular level.
Administration of any calcium salt induces a manifest increase in local alkalosis (Fig. 128) of the second day wound crust pH. It appeared interesting that in bone lesions, especially in bone cancer metastases, the off balance type A is characterized by an osteolytic process, the D type by an osteoplastic one. The local acidosis present in lesions with an A type of offbalance explains the mobilization of calcium in these osteolytic processes. Ca is deposited in important amounts in metastases with a type D offbalance, a fact which can be related to the local alkalosis resulting from the abnormal metabolism. This alkalosis represents a condition favoring the precipitation of calcium. Indirectly, the deposit of calcium in bone metastases appears to correspond to the D pattern of tissular abnormality. Calcium has a D inducing activity even in this case.
With calcium excreted in excess through the urine, the problem of calcium pharmacology in the A type of cancer is related to the form in which it acts at the cellular level, which appears qualitatively impaired. As the quantitative decrease must be considered to be a consequence of qualitative insufficiency and not a general quantitative deficiency, the problem is not to provide calcium but to find a way to insure better utilization at the cellular level. It is for this reason that administration of most calcium salts does not influence the evolution of experimental or clinical cancer, but has a preventive effect upon the induction of tumors through carcinogens. Administered after the injection of the carcinogen, calcium appears to reduce the percentage of positive results. Administered after the tumors have appeared, the influence is minimal or nil.
As we have mentioned above, an excessive calcium excretion is, in itself, sufficient to indicate the existence of a deficiency in calcium utilization at the cellular level without a calcium deficiency in the organism. The therapeutic indication is for agents able to influence the fixation of calcium at the cellular level. Fatty acids which change the cellular metabolism so as to induce local alkalosis, have appeared to be the most active agents. Testosterone, and calciferol have appeared helpful but not nearly as active as fatty acids.
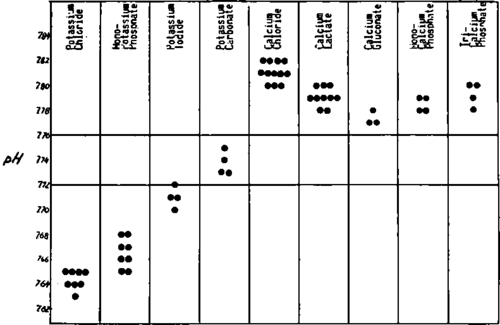
Fig. 128. The influence exerted by two elements, potassium and calcium, upon the second day wound crust pH shows a frank tendency toward acidification for potassium and alkalinization for calcium. For different salts, the differences result from the unequal influence exerted by the anion which works additively to that of the cation.
While high urinary excretion of Ca indicates a type A offbalance, low or no urinary excretion can result either from an excessive cellular utilization of calcium or from a type D offbalance at the cellular level. Other analyses can be used to indicate the probable occurrence. With calcium excretion low and other analyses indicating type D offbalance, all the chances are that the low excretion is part of the D offbalance. If, on the contrary, only the Ca excretion is low and the other values correspond to A type of offbalance, the probability is a quantitative lack of calcium in the organism. This can be corrected by the administration of calcium parenterally or orally in any absorbable form. Low excretion as well as some symptoms can be overcome in a short time through the administration of sufficient calcium. In the opposite type of case, with metabolic calcium retention, administration of calcium will induce an increase in the intensity of symptoms.
Excess of calcium in the urine thus corresponds to lower values at the cellular level and need for fixation of calcium. This indicates again that the problem is not the amount of calcium present but the deficiency in its utilization.
Continue to:


